May 13, 2024
📰 FEATURE STORY
Is the AstraZeneca vaccine disclosure significant?
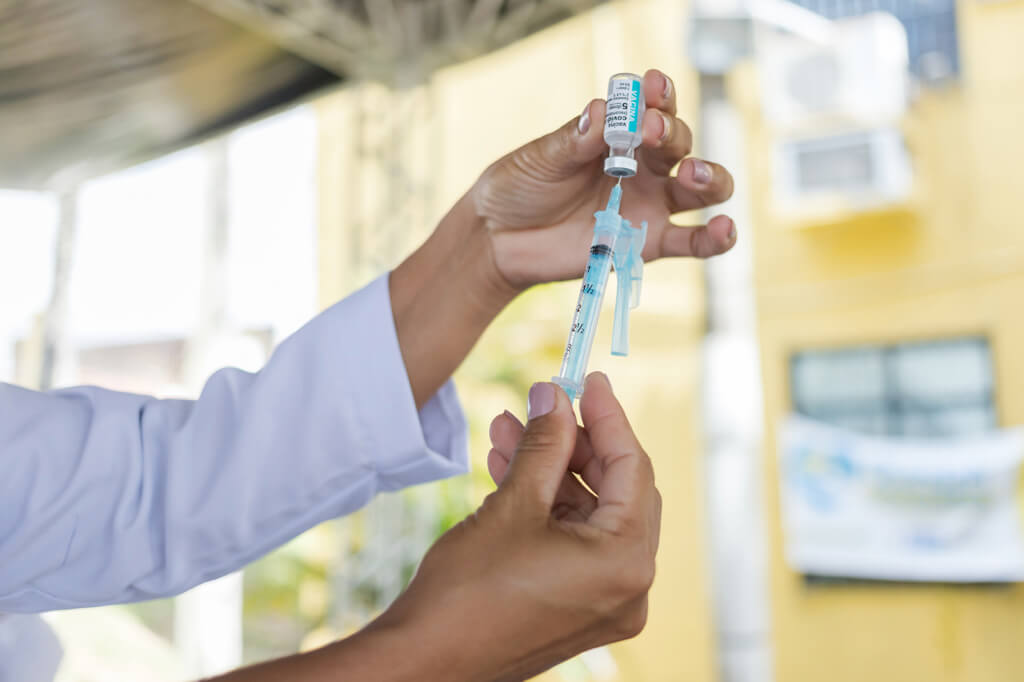
This time, a couple of years ago, things were beginning to look a little better. Countries were lifting lockdowns and travel restrictions, and we had effective Covid-19 vaccines. Those vaccines came in record time. One of the companies that gave us a vaccine was the British-Swedish company AstraZeneca. It was called Covishield.
The company is now in the news related to the vaccine but for the wrong reasons. It admitted that Covishield could cause a side effect called thrombosis with thrombocytopenia syndrome (TTS). That’s left many people who took the vaccine worried. Is this news significant, or is it being blown out of proportion?
Context
As the coronavirus began to spread, research teams at the Oxford Vaccine Centre and AstraZeneca decided to come together to develop a vaccine that could protect people. This was in early February 2020. Only a year later, the vaccine was administered to the British public and other countries with full backing from the World Health Organisation (WHO).
Here’s how it was done. The Oxford team took a common cold virus that infects chimpanzees and engineered it in a way that wouldn’t trigger an infection in humans. They further remodified it so it carried the genetic blueprints for pieces of the coronavirus.
Here’s what happens when a person takes the jab. When injected, the adenovirus, a gene for the coronavirus spike protein that’s added to another virus, bumps into cells and attaches to proteins on their surface.
Once the virus is inside, the adenovirus travels to the nucleus, where the cell’s DNA is located. The adenovirus is engineered to make copies of itself. It provokes the immune system by basically switching on the cell’s alarm systems. They then begin to attack the coronavirus spike proteins.
AstraZeneca came into the picture to help with testing and large-scale manufacturing. The company already had experience manufacturing the live virus influenza vaccine.
Last month, the pharmaceutical company was sued in the UK over claims that the vaccine caused death and injury to dozens. In court documents, the company admitted for the first time that it could result in TTS. It results in people developing blood clots and a low blood platelet count. 51 cases have been filed with victims and their kin demanding damages of up to 100 million pounds.
Lawyers for the families argued that the company lied about the vaccine’s efficacy and wasn’t honest about its side effects. The company responded by saying patient safety is its highest priority, citing several regulatory standards and protocols that its vaccine had to go through and clear.
Do these claims undermine the vaccine, the development process, and its efficacy?
VIEW: Should be taken seriously
The case that perhaps started all this was that of a 47-year-old father-of-two named Jamie Scott. He was fit and healthy before he took the jab. An adverse reaction resulted in him fighting for his life. He suffered permanent brain damage. He and his wife Kate sought to get adequate compensation from the company, but they kept playing hardball.
The company and the UK government have tried to brush these developments under the carpet, but a determined group of people aren’t standing for it. In a 2021 study by scientists from Oxford University, they compared the possibility of thrombocytopenia after Covid-19 infection, and after the administration of the AstraZeneca vaccine. The conclusion was an increased risk of low platelet count in short intervals after the first dose.
The risk was so problematic that some countries, especially in Europe, suspended the AstraZeneca vaccine for a limited time. UK and Australian governments even recommended that people below 50 not take the AstraZeneca vaccine. In India, the government never really undertook any study to understand the vaccine’s adverse effects. The company has now decided to withdraw its vaccine, citing a “surplus of available updated vaccines”.
COUNTERVIEW: It’s overblown
The first thing that needs to be stated is that no vaccine against any virus is 100% safe. There are always going to be some side effects. Many people might’ve experienced mild flu-like symptoms after taking the Covid-19 vaccines. In vaccines that fight other viruses, rare side effects have been observed, but they’re still used. That’s because they’re fundamentally safe. It shouldn’t be a surprise that the AstraZeneca vaccine can have rare and adverse side effects, but it shouldn’t be a cause for panic.
The operative word here is “rare”. Data from the UK and the European Union (EU) assessed the vaccine’s safety and said the risks were low. One particular study looked at the AstraZeneca Global Safety Database in 2022 and found the rate of adverse events to be 7.5 per million vaccinated people. Another study also looked at the AstraZeneca vaccine and found the rate of severe adverse reactions at two per million.
One of the recent claims is that this is the first time the world has come to know about TTS as an adverse side effect. That’s only partly true since it’s certainly the first time this information has reached a wide audience. However, scientific papers tackled this in 2021. AstraZeneca released a “package insert” shared on its website when it rolled out the vaccine. It stated that clotting could be a rare occurrence. People just weren’t aware of it then.
Reference Links:
- The story behind the Oxford-AstraZeneca COVID-19 vaccine success – UK Research and Innovation
- How the Oxford-AstraZeneca Vaccine Works – The New York Times
- AstraZeneca admits its Covid vaccine can cause rare side effect in court documents for first time – The Telegraph
- Covishield’s ‘rare’ side-effects: In election season, dangers of politicising the vaccine – The Indian Express
- Side effects of AstraZeneca vaccine: Medicine is clear now to the courts – The Indian Express
- Report on Rare Adverse Side Effects of Covishield Causes Panic. But Should It? – The Wire
What is your opinion on this?
(Only subscribers can participate in polls)
a) The AstraZeneca vaccine disclosure is significant.
b) The AstraZeneca vaccine disclosure isn’t significant.
🕵️ BEYOND ECHO CHAMBERS
For the Right:
Why some Kashmiris who spurned ballot in the past may vote in this Lok Sabha election
For the Left:
Wealth Tax: Producing More Heat Than Light

